Advances in the clinical management of sickle cell disease (SCD) have significantly improved the life expectancy of patients. However, aging in SCD is worryingly associated with extensive end-organ damage, including nephropathy, hepatopathy, and pulmonary alterations. Continuous vaso-occlusive (VO) and inflammatory processes in the vasculature are most likely the principal cause of such lesions, and there is little evidence, to date, that long-term hydroxyurea therapy (the standard of care for SCD) reduces organ damage in SCD adults. Inhibition of P-selectin activity, using crizanlizumab, has shown some success for reducing VO crisis frequency in SCD. Furthermore, interleukin (IL)-1β blockade, in the form of canakinumab administration, was well tolerated in young patients with SCD and associated with inflammatory marker reduction. Exploring the hypothesis that a multi-target approach could potentiate the effects of biological agents such as crizanlizumab, we recently showed that acute neutralization of inflammasome-processed cytokines potentiated the effects of anti-P-selectin on tumor necrosis factor (TNF)-α-induced VO events in SCD mice.
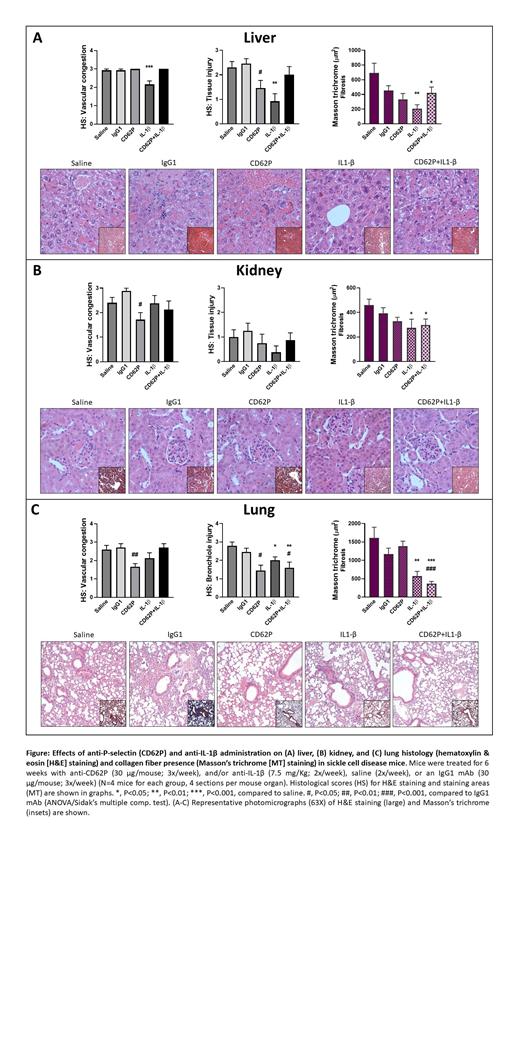
The aim of this study was to evaluate the effects of 6 weeks (wks) of anti-IL-1β and/or anti-P-selectin (CD62P) immunotherapy on inflammatory parameters and the organ histology of SCD mice. Transgenic Berkeley SCD mice (4-mo old) received ( i.p.) either anti-IL-1β monoclonal antibody (mAb) (01BSUR IgG2a; 7.5 mg/Kg; 2x/week), and/or anti-murine CD62P (RB40.34 IgG1; 30 µg/mouse; 3x/week), or an IgG1 mAb (30 µg/mouse; 3x/week) or saline (2x/week) for 6 wks (N=4-5 for each group). Prior dosing protocols determined the optimal mAb concentrations in SCD mice (data not shown). At two days after the last mAb administration, blood samples were analyzed by flow cytometry and serum stored for biomarker analysis (ELISA/colorimetric assays). Animals were then euthanized and organs were immediately collected and processed for staining with hematoxylin & eosin or Masson's trichrome (for collagen fiber staining). Histological analysis of 4 sections from each organ was performed blindly and alterations were scored qualitatively on a scale of 0 (absent) to 3 (severe).
P-selectin inhibition, but not IL-1β blockade, for 6 wks resulted in inhibition (55.4 ± 6.4% inhibition, P=0.002) of the formation of circulating neutrophil-platelet (CD45 +Ly6G +CD41 +) aggregates in SCD mice, compared to non-specific IgG1, as did the combination of both anti-P-selectin/ anti-IL-1β administration (44.4 ± 5.2% inhibition, P=0.016). In contrast, IL-1β blockade, but not P-selectin inhibition, significantly reduced the circulating levels of TNF-α, compared to the saline/IgG1 controls, after 6 wks (40.4 ± 8.9% decrease, P=0.03). No significant alterations in serum biochemical markers (aspartate/alanine aminotransferase, total/direct/indirect bilirubins) were observed following any of the immunotherapies (P>0.05). With regard to effects on organ histology (see Figure): In the liver, P-selectin inhibition reduced tissue injury, while anti- IL-1β therapy significantly reduced sinusoidal congestion, injury and fibrosis, compared to saline/IgG1-treated SCD mice. In the kidney, anti-P-selectin therapy reduced renal congestion, while anti-IL-1β therapy ameliorated fibrosis, compared to saline/IgG1-treated SCD mice. In contrast, in the lungs, anti-P-selectin therapy reduced lung congestion and cell infiltrate, and significantly reduced bronchiole injury, while IL-1β immunotherapy and P-selectin/IL-1β inhibition combination therapy abrogated bronchiole injury/ fibrosis.
As such, single immunotherapies had significant effects on multiple organs in SCD mice; P-selectin inhibition abrogated pulmonary bronchiole injury and vascular congestion, possibly mediated by reduced heterocellular aggregate formation, while IL-1β neutralization protected against liver and kidney alterations, and improved lung fibrosis, in association with reduced inflammatory cytokine production. Combination treatments, however, especially in the kidney and liver, had more complex effects. We suggest that future preclinical and clinical studies should investigate the potential of anti-inflammatory/adhesion approaches (alone or in combination) for preventing damage to multiple organs, in addition to preventing VO events.
Disclosures
Gotardo:Novartis Pharma AG: Other: Post Doctoral Fellowship Grant. Kovarik:Novartis Pharma AG: Current Employment. Bruederle:Novartis Pharma AG: Current Employment. Millholland:Novartis Precision Medicine: Current Employment. Costa:Novartis Pharma AG: Honoraria; Pfizer: Consultancy. Conran:Novartis Pharma AG: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal